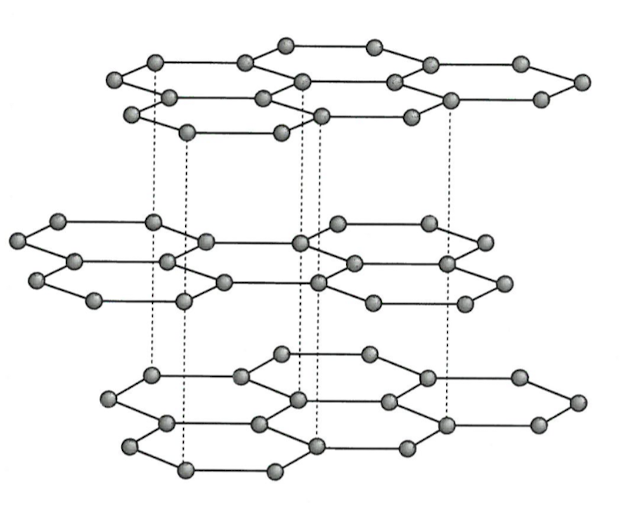
In graphite, the carbon atoms are arranged in planar layers. Within the layers, the carbon atoms are arranged in hexagons. Each carbon atom is joined to three other carbon atoms by strong covalent bonds.The picture shows the structure of graphite.The layers of carbon atoms are kept next to each other by weak instantaneous dipole-induced dipole forces.
The properties of graphite are related to its structure.
High melting and boiling points: there is strong covalent bonding throughout the layers of carbon atoms. A lot of energy is needed to overcome these strong bonds.
Softness: graphite is easily scratched. The forces between the layers of carbon atoms are weak. The layers of graphite can slide over each other when a force is applied. The layers readily flake off. This 'flakiness' is why graphite is used in pencil 'leads' and feels slippery.
Good conductor of electricity: when a voltage is applied, the delocalised electrons (mobile electrons) can move along the layers.
Graphene is a single isolated layer of graphite (Figure 5.20). The hexagonally arranged sheet of carbon atoms is not completely rigid and it can be distorted.
Graphene has some of the properties of graphite, but they are more exaggerated. For example:
• Graphene is the most chemically reactive form of carbon.
• Single sheets of graphene burn at very low temperatures and are much more reactive than graphite.
• Graphene is extremely strong for its mass.
• For a given amount of material, graphene conducts electricity and heat much better than graphite.
It has been said that 'a one square metre hammock made of graphene could support a 4 kg cat but would weigh only as much as the cat's whisker'.
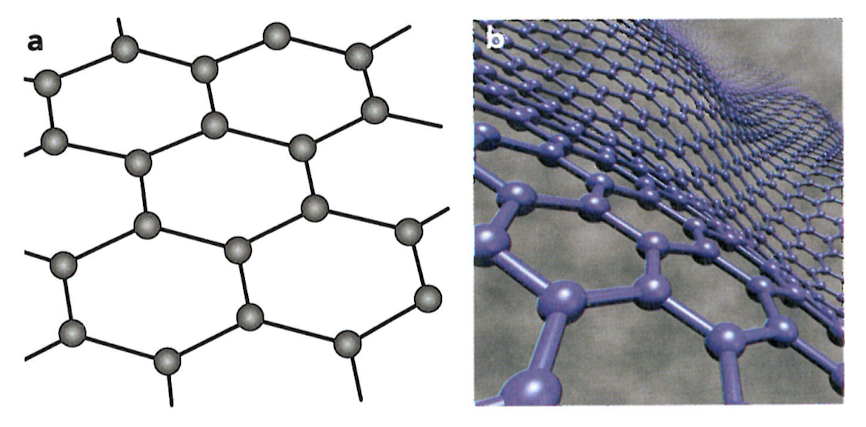
a.the structure of graphene b.'waves' in a sheet of graphene